Abstract
Acute Myeloid Leukemia (AML) is a myelogenous malignancy that frequently relapses despite complete remission (CR) and elicits non-responsiveness (refractory) to treatment. Recent studies have emphasized that acquiring resistance to treatment and/or residual subclones bearing drug persistency such as Cancer stem cells (CSCs) or Minimal residual disease (MRD) clones can lead to relapse and treatment-refractory in AML. However, due to the AML's cytogenetic clonal heterogeneity, drug persistent MRD having leukemogenic properties and its clinical relevance have not yet been fully explained. In this study, we investigate the clonal dynamics of AML MRD by pursuing their genotypic/phenotypic changes in the individual AML patient's Bone Marrow (BM) niche.
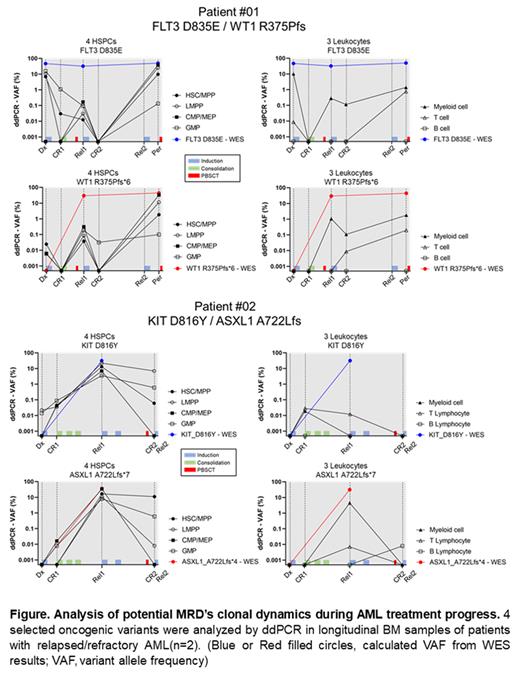
To investigate, we collected longitudinal BM samples at diagnosis (Dx), remission (CR), relapse (Rel), and treatment-refractory (Per) from two patients of relapsed/refractory AML. By bulk Whole Exome Sequencing (WES), somatic variants were analyzed, and selected four oncogenic variants (Patient #01: FLT3 D835E, WT1 R375Pfs; Patient #02: KIT D816Y, ASXL1 A722L) showing a significant change according to the treatment process. Seven blood cell fractions (4 HSPCs, Hematopoietic Stem and Progenitors: HSC/MPP, LMPP, CMP/MEP, GMP, LSC-like populations; 3 Mature leukocytes: Myeloid cells, T/B Lymphocytes) from AML patient's bone marrow samples were sorted by cell surfaces antigens. Selected AML-associated variants were detected by droplet digital PCR (ddPCR) in DNA of each sorted seven single cell fractions.
In the AML patient BM, we found that the clones with an oncogenic (or likely oncogenic) variant were distributed in not only primitive cell populations with the HSPC (LSC-like) phenotype, but also mature (differentiated) cell populations such as leukocytes. These clones were not completely disappeared even after intense AML chemotherapy, and remained in minimal quantities. In our sequential analysis, some residual clones distributed in LSC-like populations exhibited the traditionally well-known MRD's character, such as repopulating LSC proportions. Furthermore, some other residual clones only in mature populations also seemed to be played a role in functional MRD resulting in the repopulation of LSCs. However, whether these residual clones in LSC-like populations or mature populations eventually induced repopulating of LSC-like fractions during AML relapse, and particularly in the relapse before an endpoint, these clones also tended to be highly enriched in LSC-like populations by 30% (based on the VAF). While the variant clones in mature populations may be the temporal salvages of AML-MRD clones (functional MRD) to survive against treatment, the variant clones in LSC-like populations might be the AML-MRDs practically contributing to AML recurrence and treatment-refractory.
This approach suggests the methodology for specifying AML-MRD and pursuing its clonal evolution according to the treatment progress. This will help to improve AML risk stratification and the diagnostic methodology, enabling the preemptive detection of AML MRD at the initial diagnosis/treatment.
Disclosures
Yoon:Chugai Pharmaceutical: Consultancy; Roche-Genetech: Research Funding; Celgene: Consultancy; Astellas Pharma: Consultancy; Janssen Pharmaceutical: Consultancy; Takeda: Consultancy; Kyowa Kirin: Research Funding; Novartis: Consultancy; Amgen: Consultancy; Yuhan Pharmaceutical: Research Funding; Tikaros: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal